Heating and cooling curves provide invaluable insights into the thermal behavior of materials, unveiling the intricacies of phase transitions and energy changes. As we delve into the depths of heating cooling curve worksheet answers, we embark on a journey to decipher the language of thermodynamics, unraveling the mysteries that govern the physical transformations of matter.
The shape of a heating or cooling curve serves as a roadmap, guiding us through the sequential stages of energy absorption and release. Enthalpy and entropy, the driving forces behind these transitions, shape the characteristic contours of these curves, revealing the underlying energetic landscape.
1. Heating and Cooling Curve
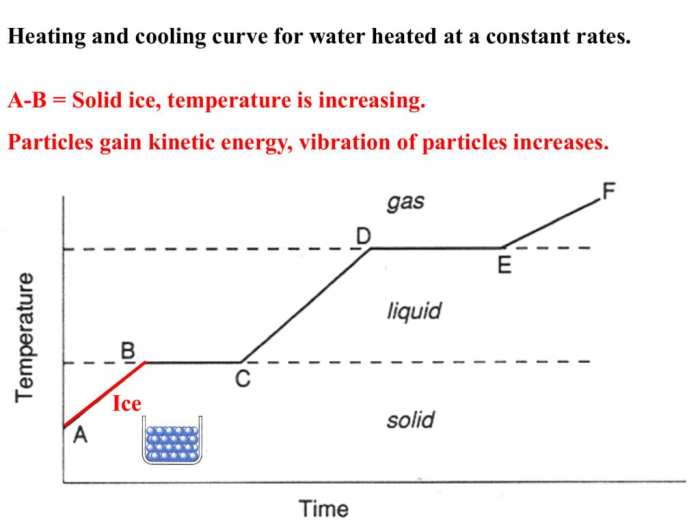
A heating and cooling curve is a graphical representation of the temperature change of a substance as it is heated or cooled. It provides valuable information about the physical and chemical changes occurring during the process.
The shape of a heating and cooling curve is typically divided into distinct stages, each representing a specific change in the substance’s state or properties.
Stages of a Heating and Cooling Curve, Heating cooling curve worksheet answers
- Initial heating:The temperature rises gradually as the substance absorbs heat.
- Melting:A plateau is observed as the substance changes from a solid to a liquid, absorbing heat without a temperature increase.
- Boiling:Another plateau is observed as the substance changes from a liquid to a gas, again absorbing heat without a temperature increase.
- Cooling:The temperature decreases gradually as the substance releases heat.
- Freezing:A plateau is observed as the substance changes from a liquid to a solid, releasing heat without a temperature decrease.
- Condensation:A plateau is observed as the substance changes from a gas to a liquid, releasing heat without a temperature decrease.
2. Enthalpy and Entropy Changes
Enthalpy (H) and entropy (S) are thermodynamic properties that describe the energy and disorder of a system, respectively. Changes in these properties are closely related to the shape of a heating and cooling curve.
During heating, the enthalpy of the substance increases as it absorbs heat, while the entropy increases due to the increased molecular motion and disorder.
During cooling, the enthalpy decreases as the substance releases heat, while the entropy decreases due to the reduced molecular motion and increased order.
The significance of enthalpy and entropy changes in chemical reactions is that they determine the spontaneity and equilibrium of the reaction.
3. Phase Transitions
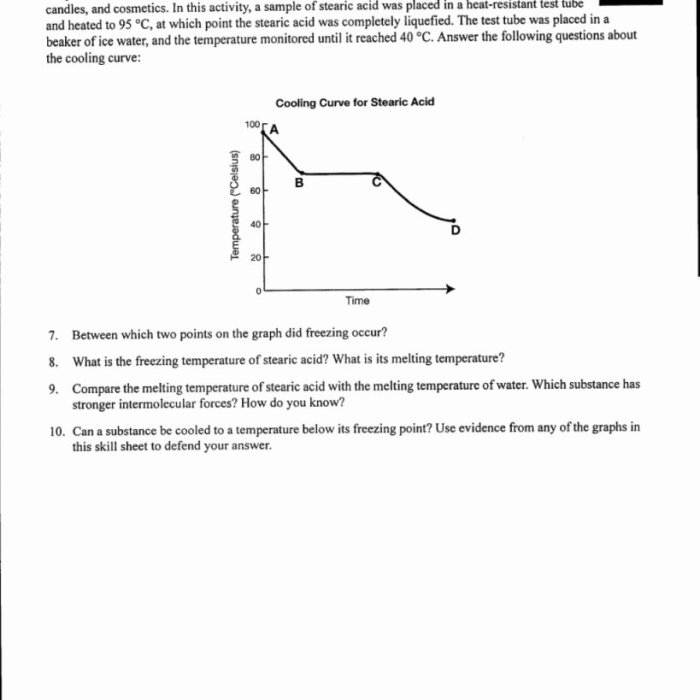
Phase transitions are physical changes in which a substance changes from one state of matter to another, such as from solid to liquid or from liquid to gas.
Phase transitions are important in heating and cooling curves because they represent specific points where the enthalpy and entropy of the substance change significantly.
Types of Phase Transitions
- Melting:Solid to liquid
- Freezing:Liquid to solid
- Boiling:Liquid to gas
- Condensation:Gas to liquid
- Sublimation:Solid to gas
- Deposition:Gas to solid
4. Applications of Heating and Cooling Curves: Heating Cooling Curve Worksheet Answers
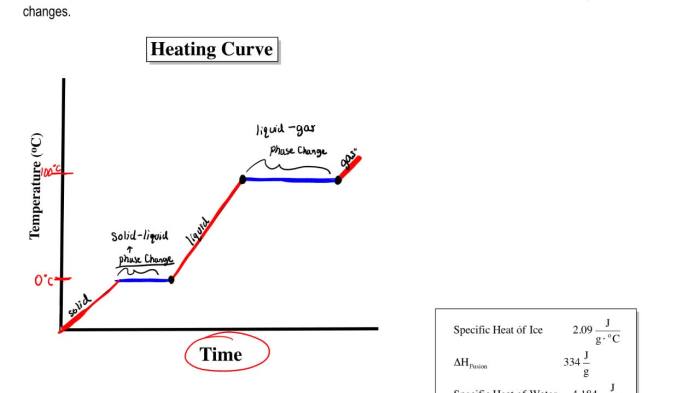
Heating and cooling curves are widely used in various fields, including:
- Chemistry:To identify substances, study reaction kinetics, and determine thermodynamic properties.
- Materials science:To analyze the thermal properties of materials, such as melting points, boiling points, and phase transitions.
- Engineering:To design and optimize processes involving heat transfer, such as in heating and cooling systems.
Limitations of heating and cooling curves include their inability to provide information about the rate of reactions or the mechanisms involved.
Essential Questionnaire
What is the significance of the plateaus in a heating or cooling curve?
Plateaus indicate phase transitions, where the temperature remains constant while energy is absorbed or released during the transformation from one phase to another.
How can heating and cooling curves be used to determine the purity of a substance?
Impurities alter the shape and temperature of phase transitions, providing clues about the substance’s composition.
What are the limitations of heating and cooling curve analysis?
Assumptions about sample homogeneity, equilibrium conditions, and heat transfer effects can introduce uncertainties.